Levamisole Hydrochloride Reference High-Purity Reference Materiel for Sale
Original price was: $89.00.$69.00Current price is: $69.00.
+ Free Shipping- Product Code: L-D25003X
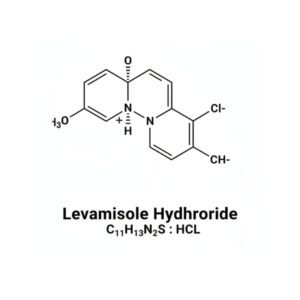
- Chemical Name: Levamisole Hydrochloride
- Category: Drug Impurity Standard
- CAS Number: 16595-80-5
- Molecular Formula: C₁₁H₁₃ClN₂S
- Molecular Weight: 240.75 g/mol
- Purity: ≥ 95%
- Available Package Size: 25 mg
- Storage Conditions: Room Temperature
- Special Requirements: None
1️⃣ Product Description
Product Name: Levamisole Hydrochloride Reference Standard
CAS Number: 16595-80-5
Product Code: L-D25003X
Category: Drug Impurity Standard
Short Overview:
Levamisole Hydrochloride (CAS 16595-80-5) is a high-purity drug impurity reference standard (≥ 95%) used for pharmaceutical quality control, impurity profiling, and analytical method validation.
It is ideal for HPLC and LC-MS testing of antiparasitic formulations and related compounds.

Information:
| Parameter | Value |
|---|---|
| Molecular Formula | C₁₁H₁₃ClN₂S |
| Molecular Weight | 240.75 g/mol |
| Purity | ≥ 95 % |
| Storage Conditions | Room Temperature |
| Package Size | 25 mg |
| Documentation | COA & SDS supplied |
| Compliance | ISO/IEC 17025 & GMP procedures |
💡 Stable at ambient temperature and classified as non-hazardous for international shipping.
2️⃣ Features & Benefits / Technical Information
Features & Benefits
- ✅ Analytical-grade purity (≥ 95%) verified by LC-MS and HPLC
- ✅ Supplied with COA & SDS for traceability and compliance
- ✅ Produced under ISO/IEC 17025 and GMP-certified processes
- ✅ Stable at room temperature with long shelf life
- ✅ Ideal for impurity profiling and method validation studies
- ✅ Custom sizes available with global delivery
Technical Information
| Parameter | Details |
|---|---|
| Structural Formula | (Available upon request or displayed as image) |
| Appearance | White to off-white crystalline powder |
| Storage Conditions | Room Temperature (dry and sealed environment) |
| Shelf Life | 24 months from manufacture date |
| Analytical Techniques | HPLC, LC-MS, GC |
| Category | Drug Impurity Standard |
📎 Each batch is validated by HPLC and LC-MS for purity and structural identity prior to release.
3️⃣ Applications
Levamisole Hydrochloride reference standard is commonly used in:
- Pharmaceutical Quality Control: Identification and quantification of impurities in antiparasitic drug products.
- Stability Testing: Detection of degradation and oxidation products under various storage conditions.
- Analytical Method Development: Calibration and validation of HPLC and LC-MS methods.
- Research & Development: Studies of drug metabolism and pharmacokinetics.
💡 Ensures precise, reproducible results and traceability across regulated pharmaceutical laboratories.
4️⃣ Frequently Asked Questions (FAQs)
Q1. What analytical methods are compatible with Levamisole Hydrochloride standards?
HPLC, LC-MS, and GC methods are suitable for both qualitative and quantitative impurity testing.
Q2. Do I receive COA and SDS with my order?
Yes, each shipment includes a Certificate of Analysis and Safety Data Sheet.
Q3. Can I request bulk or custom package sizes?
Yes, packaging from 25 mg to 1 g or more is available upon request.
Q4. How should I store this product?
Store tightly sealed at room temperature in a dry environment away from direct sunlight.
Q5. Is this product hazardous for shipping?
No, Levamisole Hydrochloride reference standard is classified as non-hazardous for global shipping.
5️⃣ Ordering & Support
| Product Code | CAS | Size | Availability |
|---|---|---|---|
| L-D25003X | 16595-80-5 | 25 mg | ✅ In Stock |
📦 Delivery: Ships within 3–5 business days worldwide.
🧾 Includes: COA & SDS for each batch.
🌍 Global Shipping: Temperature-protected packaging available for long-distance transport.
📩 Contact Sales: sales@hipsoulab.com
📞 Customer Support: +86-755-28502380
💬 Hipsoul — Delivering purity, precision, and reliability to analytical laboratories worldwide.
You must be logged in to post a review.


Reviews
There are no reviews yet.